Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
Your Eversense Inserter will explain and perform the simple and quick steps to insert the sensor. You will be fully awake during the approximately 5-minute insertion procedure. (The entire procedure, including preparation, usually takes about 15 minutes.)
It is important to choose a site (on the upper arm) that is comfortable for you to wear the sensor and smart transmitter for up to 180 days. It is recommended to have the sensor inserted toward the back of the upper arm. Placement in this area minimizes the chance of the sensor and smart transmitter being bumped by doorways, walls or other narrow passages. It is recommended to avoid areas with loose skin, scars, tattoos, nerves, or blood vessels that could be incised during the procedure. It is recommended to alternate arms for subsequent insertion sites.
Step 1: Site preparation – the insertion site will be cleaned, disinfected, then anesthetized using lidocaine.
Step 2: Incision – a small (less than 1 centimeter) incision will be made at the insertion site.
Step 3: Sensor insertion – a subcutaneous pocket will be created under the skin and the sensor will be inserted in this pocket.
Step 4: Site closure – the incision will be closed with Steri-Strips™. Sutures are not usually required.
Step 5: Sensor and smart transmitter linking – link the sensor and smart transmitter to begin the 24-hour Warm-Up Phase.
Note: After insertion, link the smart transmitter and the sensor and then allow the incision site to heal 24 hours before replacing the transmitter.
The sensor requires 24 hours to stabilize within the insertion site, this period is known as the Warm-up Phase. After the first 24 hours of sensor insertion, position and secure the smart transmitter over the sensor and ensure you have a connection. Then you can perform your Initialization Phase calibration of 4 fingerstick blood glucose tests to start getting glucose readings.
Homopolymer polymethylmethacrylate (PMMA), Hydroxyethylmethacrylate (HEMA) based Hydrogel, Platinum, Silicone, Dexamethasone Acetate, epoxy 301-2
No, however data from the clinical study showed all users were prompted to switch to 1 calibrations/day for the first time within about 10 days after day 21.
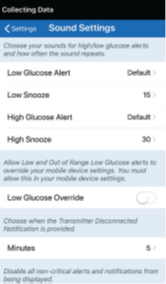
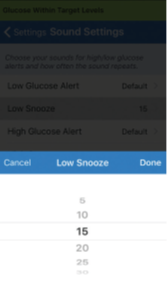
By setting the snooze alert, you can set how often an alert repeats after you have received a Low Glucose and High Glucose alert. The Low Glucose alert repeat interval can be adjusted from 5 minutes up to 30 minutes. The High Glucose alert repeat interval can be adjusted from 15 minutes up to 180 minutes.
- Tap Menu > Settings > Sound Settings to display the SOUND SETTINGS screen.
- Tap each snooze alert to set how often the alert repeats. Tap Done when complete.
The Eversense® Charging Accessory Pack includes:
- Charging Cradle (that is used to charge the Smart Transmitter)
- Charging Cable
- Wall Plug
During times when you need extra protection during heavy perspiration, we suggest purchasing Grifgrips™ (www.grifgrips.com) adhesive enhancers designed for the Eversense® Smart Transmitter, or athletic tape (e.g., KT Tape®), to help keep the transmitter securely in place in situations where heavy sweating may occur.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1