Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
You can see your last calibration in one of two ways:
In the system Event Log (Menu → Event Log), the value and time of your most recent blood glucose calibration will be listed, and will be indicated by a red blood drop icon. If helpful, you can sort for only blood glucose entry events by tapping on the blood drop icon in the sort bar at the top of the screen.
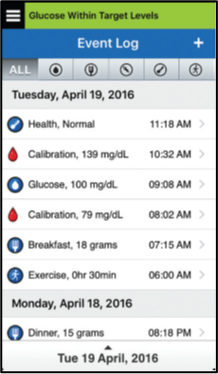
Secondly, your most recent successful calibration will be visible on your My Glucose Home Screen with a red blood drop.
The Eversense® E3 Sensor Kit includes:
- Pouch with the Sensor inside of a sensor holder
The Eversense® E3 CGM System has not been tested using insertion sites other than the upper arm.
- Width: 37.6 mm
- Length: 48.0 mm
- Thickness: 8.8 mm
You can view a snapshot of the Home screen on your iOS device if you add the Eversense® App widget to your widget page. For information on managing widgets, consult your iOS device user guide.
You can view a snapshot of the Home screen on your Android device if you swipe down on your device to show the notification tray. The app must be running in the background. This will show you the latest glucose.
You do not need to change the battery. The Eversense® E3 Smart Transmitter has a rechargeable battery and is reusable for up to one year. After one year, the warranty expires and the transmitter should be replaced with a new transmitter.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1