Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
The sensor has a silicone ring that contains a small amount of an anti-inflammatory drug (dexamethasone acetate). For more information regarding dexamethasone acetate please contact your health care professional.
If you are concerned that you may be experiencing an adverse effect, contact your health care professional.
Once a new sensor is inserted by your health care professional and paired with the transmitter using the Eversense® App, it will start the 24-hour warm-up phase. Once the 24-hour warm-up phase is completed, you will begin the Initialization Phase.
Initialization Phase (after 24-hour Warm-Up Phase)
During this phase, 4 finger stick blood glucose meter tests are required.
- The 4 calibration tests must be spaced 2 to 12 hours apart, and all 4 tests must be completed within a 36 hour period.
– 1st calibration = 24 hours after sensor insertion.
– 2nd calibration = 2 to 12 hours after 1st successful calibration.
– 3rd calibration = 2 to 12 hours after 2nd successful calibration.
– 4th calibration = 2 to 12 hours after 3rd successful calibration.
- Glucose readings will start displaying in the app a few minutes after the 2nd calibration is successfully completed.
Eversense E3 has demonstrated excellent accuracy when you need it most - we’ve demonstrated 99% of high (180 mg/dL) and 94% of low (70 mg/dL) glucose events1 detected correctly and essentially no compression lows.2
1. Senseonics. (2023) Eversense E3 Continuous Glucose Monitoring System User Guide. LBL-6002-01-001_Rev C
2. Christiansen MP et al. A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II. DIABETES TECHNOLOGY & THERAPEUTICS 2018; 20(3):197-206
Lithotripsy:
The use of lithotripsy is not recommended for people who have an inserted sensor because the effects are unknown.
Diathermy:
DO NOT use diathermy on people who have an inserted sensor. Energy from the diathermy can transfer through the sensor and cause tissue damage in the insertion area.
Electrocautery:
The use of electrocautery near the inserted sensor may damage the device. DO NOT use electrocautery near the sensor.
- Go to the Apple App Store or Google Play™ and search for “Eversense NOW”.
- Tap the Eversense NOW icon, download and install the app to your smart device.
- When prompted, tap “Allow” in order to receive glucose related alerts from the Eversense® CGM users on your list.
Precaution: If you do not allow notifications from the Eversense® NOW App, you will not receive glucose related alerts from Eversense® CGM users.
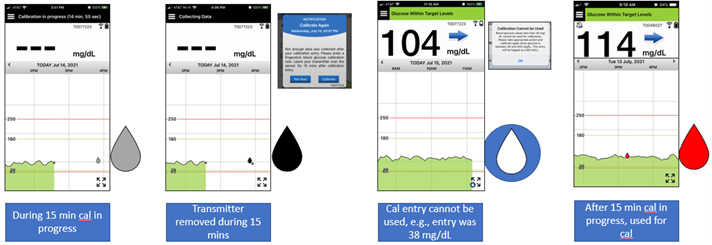
Yes, to make it clearer to users about the status of their calibration entry, during the 15 minutes after a calibration is entered and calibration by the system is in progress, the blood drop on the Home Screen trend graph will be grey. Once calibration is accepted, the drop will turn red. If the transmitter is removed during the 15 minutes that calibration is in progress, the blood drop will be black.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1