Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
It takes approximately 15 minutes to fully charge a smart transmitter when plugged into a wall outlet. It may take longer if charging via a computer USB port or when the battery is empty.
The Eversense® E3 CGM System has not been tested in the following populations: women who are pregnant or nursing, people under the age of 18, critically ill or hospitalized patients, people receiving immunosuppressant therapy, chemotherapy or anti-coagulant therapy, those with another active implantable device, e.g., an implantable defibrillator (passive implants are allowed, e.g., cardiac stents), those with known allergies to or using systemic glucocorticoids (excluding topical, optical or nasal, but including inhaled).
Areas where you can customize app settings include:
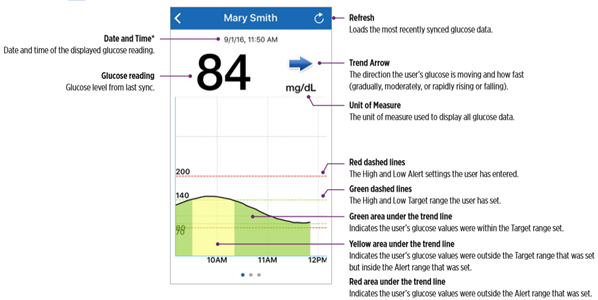
- Glucose – glucose levels and change rates that will trigger an alert.
- Calibration Reminders – optionally set calibration reminder times.
- System – identifies or lets you enter personalized information about your system.
- Sound Settings – change the sounds for some glucose alerts, set snooze times and Do Not Disturb.
- Temp Profile – set a temporary glucose profile.
- Glucose Levels
The Eversense® E3 CGM System is designed to provide alerts on your smart transmitter and mobile device when your glucose level has reached the alert levels you set. You will decide the settings for your glucose alerts, targets, and rates of change based on input from your health care professional.
Eversense 365 is the only CGM that lasts for a year - see how we compare!
A blood glucose meter is required for calibrating the CGM System, and to make treatment decisions under certain conditions. See Understanding Treatment Decisions with CGM in the Eversense® E3 CGM User Guide for more information.
Calibration is used to ensure the accuracy of the CGM system.
Pay close attention to your glucose values, symptoms, and trends. If your symptoms are different than the sensor glucose values or what the alert indicates, confirm your glucose value with a blood glucose meter test before making a treatment decision.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1