
How to upgrade your Eversense Smart
Transmitter
Get Ready First (Before You Upgrade)
Before you upgrade the firmware on your Smart Transmitter using the Eversense 365 App, you need to do a few important things. These steps will help make sure the upgrade goes smoothly:
- Check for Eversense 365 App Updates: Open your app store and check if there’s a new version of the Eversense 365 App. If there is, download it.
- Charge Your Transmitter: Make sure your Smart Transmitter is fully charged before you start the upgrade.
- Choose a Convenient Time: Upgrading can put your system into the Initialization Phase, which requires four fingerstick calibrations.
NOTE: After Initialization, your system will return to the calibration phase it was in before the upgrade.
Upgrade the Firmware in your Smart Transmitter
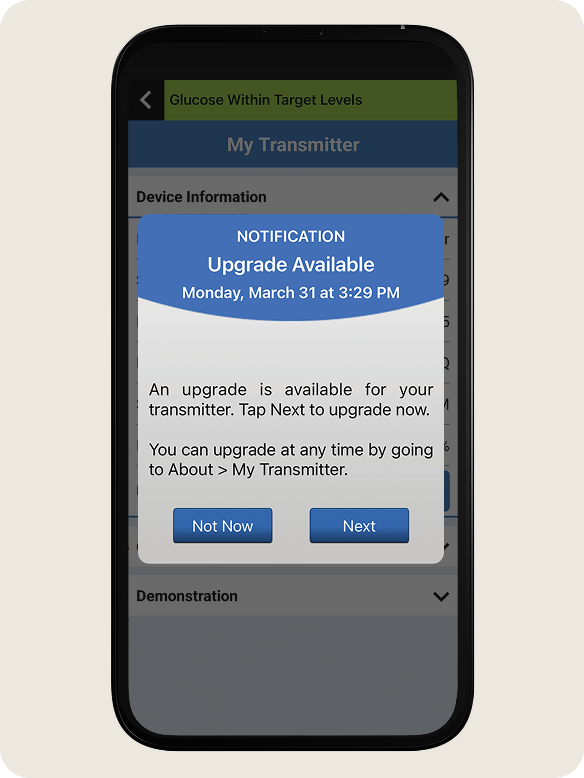
Upgrade Notification: When a firmware upgrade is available for your transmitter, you will receive an Upgrade Available Notification on your app.
STEP 1:
- To begin the firmware upgrade, tap Next.
- If you are unable to upgrade at this time, tap Not Now.
- You will be reminded of your upgrade periodically but you are able to access the firmware upgrade when you are ready.
- Go to the Main Menu > About > My Transmitter
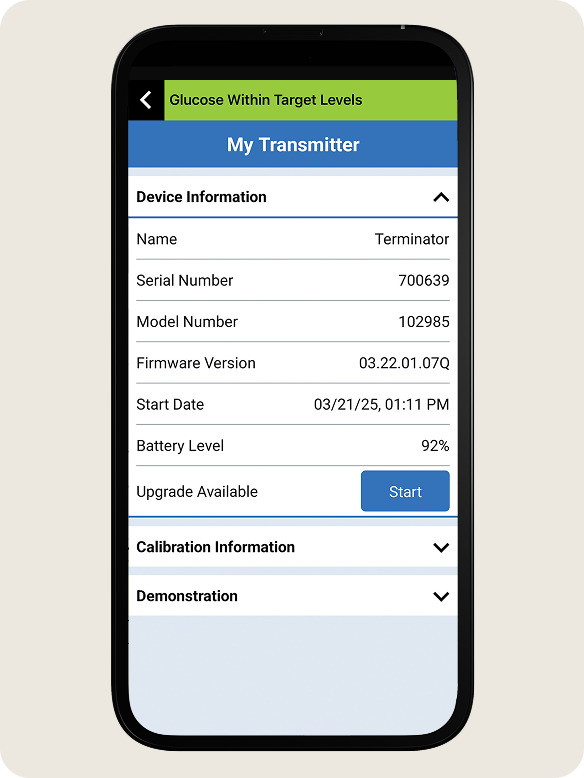
STEP 2:
- The system will display the new firmware version when the upgrade is ready to install.
- Tap Continue when prompted.
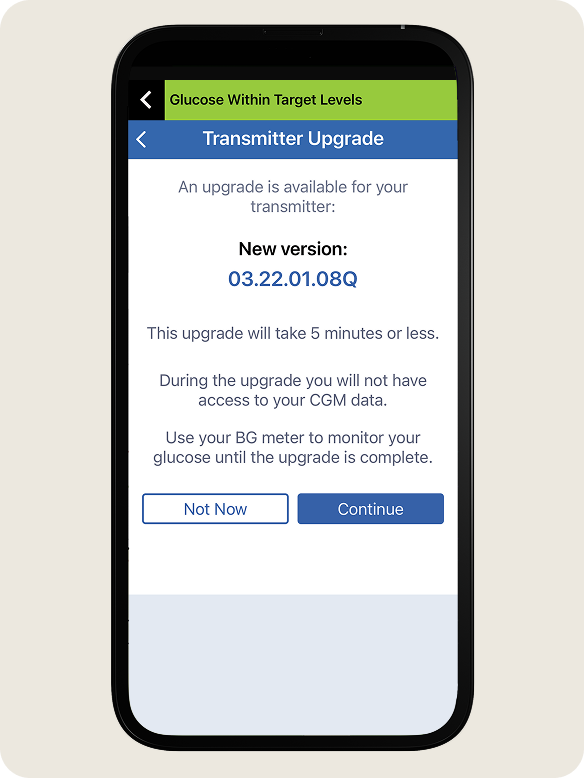
The system may not be able to begin the firmware upgrade if the transmitter battery is too low, a calibration is in progress, or if the transmitter is disconnected.
STEP 3:
- Review the changes in the upgraded transmitter version.
- Check the box to confirm you have reviewed and understand the changes.
- Tap Start Upgrade.
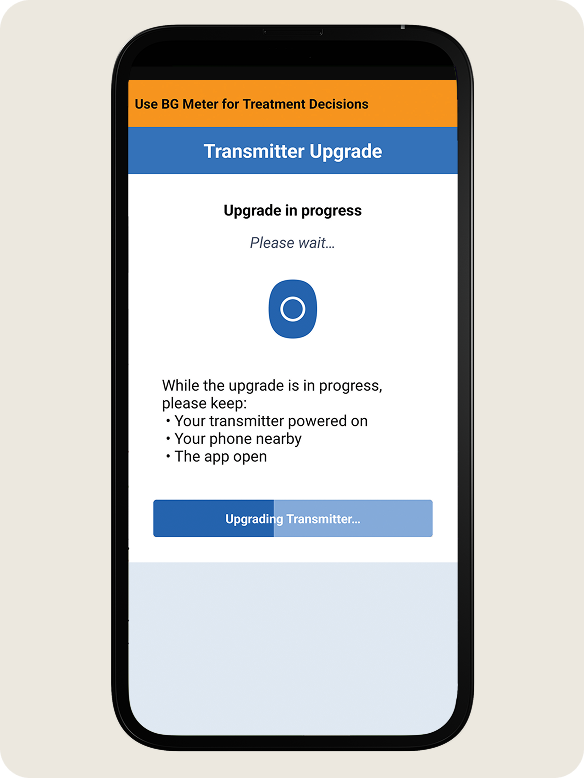
- DO NOT turn off your transmitter, move away from your phone or close the app during this time.
- You will see a progress bar while the upgrade is taking place.
- During the upgrade process the transmitter will reconnect automatically.
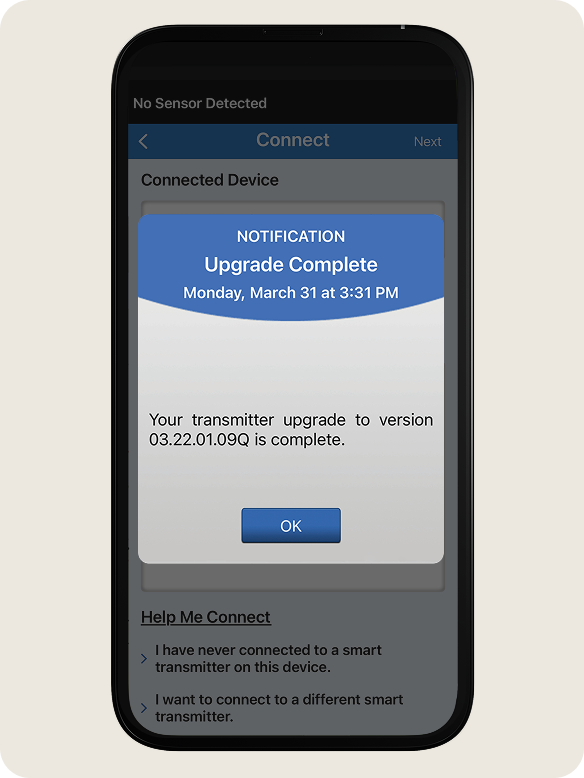
- Once the upgrade is complete, tap OK.
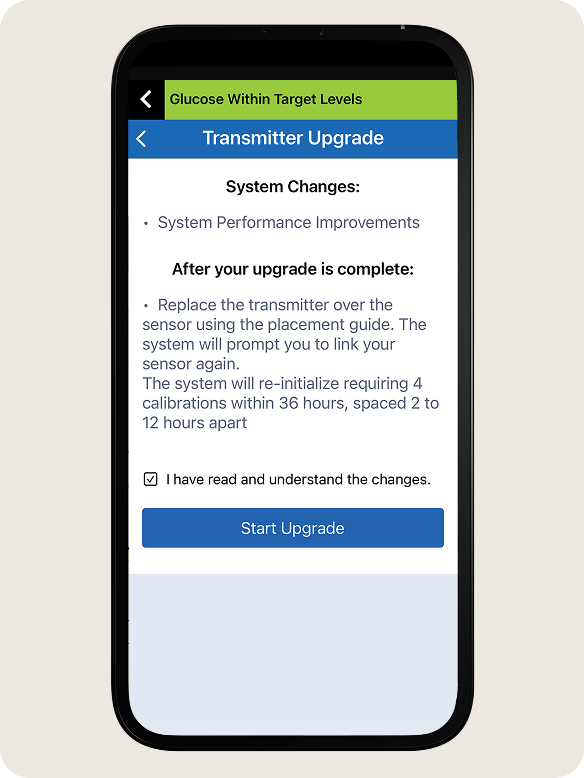
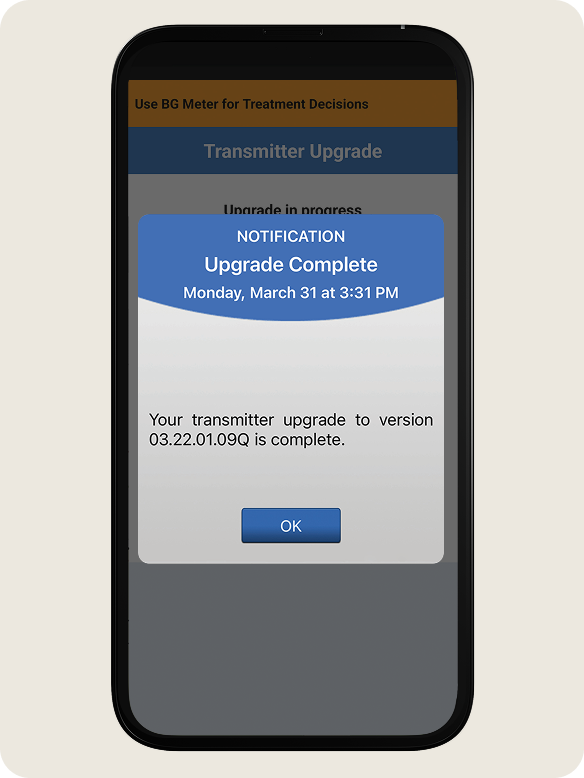
NOTE: If your transmitter does not automatically reconnect, see Next Section TRANSMITTER RECONNECT
Transmitter Reconnect
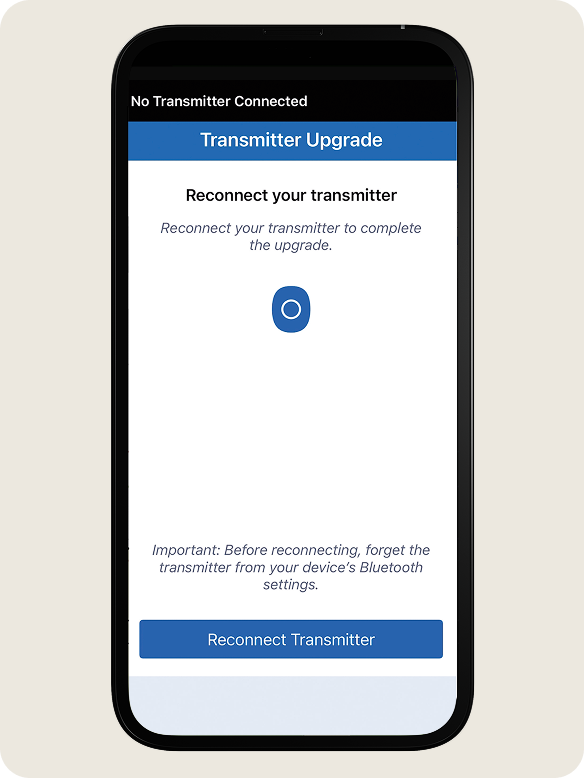
During some upgrades, you may be prompted to reconnect your transmitter.
IMPORTANT: Before reconnecting, FORGET the transmitter from your device’s Bluetooth settings
STEP 1:
When prompted, tap Reconnect Transmitter.
STEP 2:
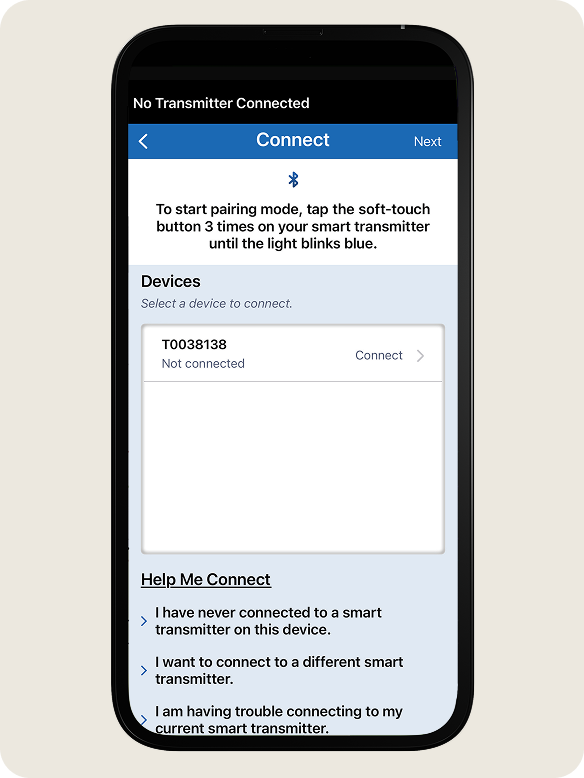
- With the smart transmitter turned on, press the soft touch button three times to start pairing mode.
- The LED will blink blue to indicate the smart transmitter is in pairing mode.
NOTE: Your mobile device must be connected to the internet in order to pair with the smart transmitter.
- On the Connect screen, tap your smart transmitter serial number.
- Your smart transmitter serial number can be found on the back of the smart transmitter.
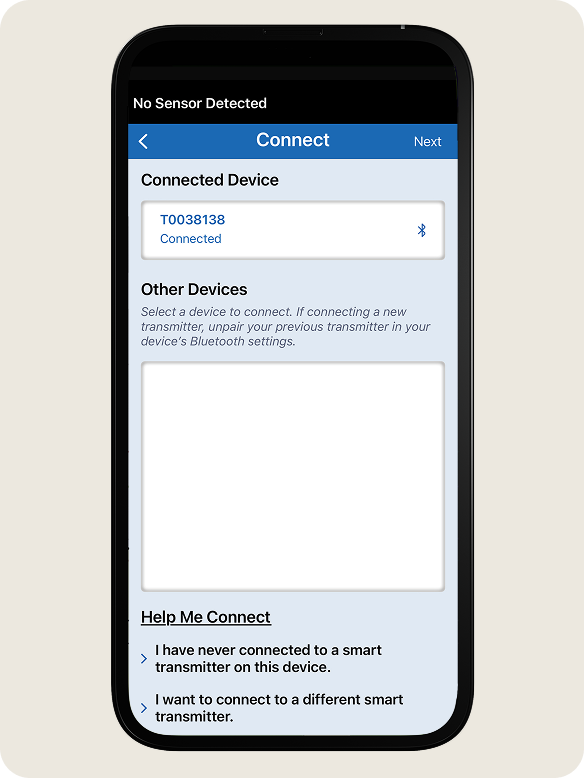
- The smart transmitter serial number and Connected will be displayed under Connected Devices once the pairing is complete. You will be redirected to the My Glucose screen.
- Once you receive the Upgrade Complete notification, tap OK. You can now use the system as your normally would.
Contact Us
If you have any questions or need assistance with your firmware upgrade, our Customer Service team is here to help. You can reach us Monday – Sunday, 8am-12am EST at 1-844-736-7348 (844-SENSE4U).
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001704 Rev 1