Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
Manual BG entries cannot be edited. Meals, Insulin, Health, and Exercise icons may be edited by going to the Event log and tapping on the entry.
An event icon can be modified or hidden after it was previously saved. From the Event Log, click on the event icon you would like to modify. Once the changes are made, tap Save.
To remove an event icon, select the event icon you would like to modify, scroll down to the bottom of the page and select “Hide Event”. This will remove the event icon from the home page.
Manual BG Entry:
- Can be hidden
- Cannot be edited
- Cannot be deleted
Events:
- Can be hidden
- Can be edited
- Cannot be deleted
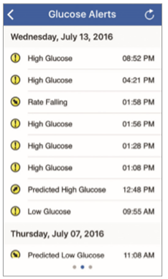
To view the Eversense® CGM user’s Glucose Alert History, swipe your screen to the left from the trend graph.
The Alert History screen will list the last 20 glucose-related alerts that the user received in their Eversense® CGM App.
These alerts monitor events such as the rising or falling of glucose rates as well as low or high, and predicted low or high, glucose values (hypoglycemia or hyperglycemia).
Note: You will only receive predicted high and low glucose alerts if the Eversense® CGM user has predictive alerts enabled.
While the Eversense® E3 Sensor is intended to be removed at end of sensor life, it has undergone biocompatibility testing and meets the requirements of a permanent implant, and therefore it should not cause a biocompatibility concern if left in a patient. However, the sensor will not provide data beyond 180 days and you will need to make arrangements to have it removed.
A compatible smart device is required in order to use the Eversense E3 CGM. Please see our Compatibility page for a list of compatible devices.
To clean your smart transmitter, wipe it down with a water dampened cloth. Reuse after drying completely.
When traveling, your smart transmitter and sensor are safe to go through airport security without removing them. You may inform security that you have an implanted medical device.
Your smart transmitter will automatically sync to your smartphone’s current time and date when time zones are changed. You should allow for automatic syncing or manually change the time zone (not the time) if needed.
The Eversense® E3 CGM System is safe for use on U.S. commercial airlines. The Eversense® E3 Smart Transmitter is a Medical Portable Electronic Device (M-PED) with emission levels that meet FAA mandates for use in all modes while in flight. (Reference FAA Advisory, Circular #21-16G, dated 6.22.2011.) To use, turn your mobile device’s Bluetooth® feature on after you have put your mobile device in airplane mode. For flights outside the US, follow local security regulations for use of medical devices in flight.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1