Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
Pinch in and out on the screen to display different day/time ranges on the trend graph. You can view as few as 3 hours or up to 3 days of information on the My Glucose screen.
You can view the trend graph in either portrait or landscape mode. In landscape mode, there are shortcut buttons to see 7, 14, 30 and 90 day views and to email the graph.
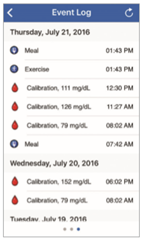
To view the Eversense® CGM user’s Event Log, swipe the screen to the left from the Alert History.
The Event Log lists the 20 most recent events logged by the CGM user. The Event Log shows information entered by the Eversense® CGM user such as calibrations, meals, exercise, blood glucose values, insulin levels, health, and exercise. If the user has not entered any Events this log will be empty.
The Eversense® E3 Sensor has a Warm-Up Phase, which is the period the sensor requires to adjust after the sensor has been inserted and before calibrations. The Warm-Up Phase lasts for 24 hours after the sensor is linked. Once the 24 hour period has passed, the Eversense® App will prompt the user to perform a calibration.
The Eversense® E3 CGM System provides readings within 40 – 400 mg/dL range every 5 minutes.
To turn the smart transmitter ON, press and hold the power button for about five seconds.
- The smart transmitter will vibrate once.
- Release the power button and the LED will blink once indicating the power is ON.
At any time, you can press the power button once to see if the smart transmitter is ON. If the LED appears, the smart transmitter is ON. If no LED appears, the smart transmitter is OFF.
It would be best not to shave the insertion site immediately before inserting the Sensor, as the skin may become sensitive or slightly irritated from shaving. Your health care professional will determine if hair removal is necessary prior to insertion.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1