Your Questions, Answered
We’ve assembled the most frequently asked questions about Eversense into one place. Got a question that you can’t find the answer to? Contact us.
The system is indicated (approved) for use to replace fingerstick blood glucose measurements for diabetes treatment decisions.
Pay close attention to your glucose values, symptoms, and trends. If your symptoms are different than the sensor glucose values or what the alert indicates, confirm your glucose value with a blood glucose meter test before making a treatment decision.*
To make a treatment decision, the user should consider:
- Status bar information.
- Current sensor glucose value – the current glucose value should be displayed in black.
- Trend arrow – a trend arrow should be displayed.
- Recent trend information and alerts.
When to NOT make a treatment decision:
- No glucose value is displayed.
- No trend arrow is displayed.
- Your symptoms do not match the glucose information displayed.
- The current sensor glucose value is displayed in grey.
- The status bar is displayed in orange.
- You are taking medications of the tetracycline class.

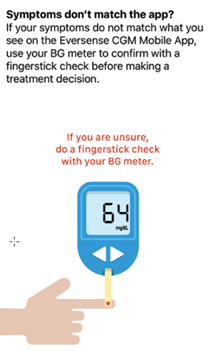

* If you have any uncertainly in how or when to make treatment decisions, always speak with your healthcare professional.
Note: Always refer to the glucose information on your Eversense CGM App on your smartphone to make treatment decisions. Do not use a secondary display like the Apple Watch or Eversense NOW.
To log out of your Eversense® account, tap About > My Account > Log Out.
IMPORTANT: If you log out, no glucose data will be displayed on the app until you log back in using the email and password you used to create your account.
It is recommended to avoid areas with loose skin, scars, tattoos, nerves, or blood vessels that could be incised during the procedure.
| Characteristics | Description |
|---|---|
| Input/Output | 5V DC, 1A |
| Type | USB-A to USB micro-B |
| Length | 36 inches (91 cm) |
You should avoid massage therapy near the inserted sensor site. Massage therapy near the sensor site could cause discomfort or skin irritation.
The Eversense® NOW App is only intended for viewing glucose data.
Still have questions?
The Eversense team is dedicated and ready to provide the answers and support you need. Simply click the link below.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001692 Rev 1