Numbing
The provider sterilizes and numbs the area

One CGM sensor placement. One year of accurate, reliable1,2 glucose monitoring.
How simple can it be? See in our video.
One sensor placement appointment is all you need for a YEAR of reliable, accurate glucose monitoring. See what it’s like.
Watch our video for all the details.
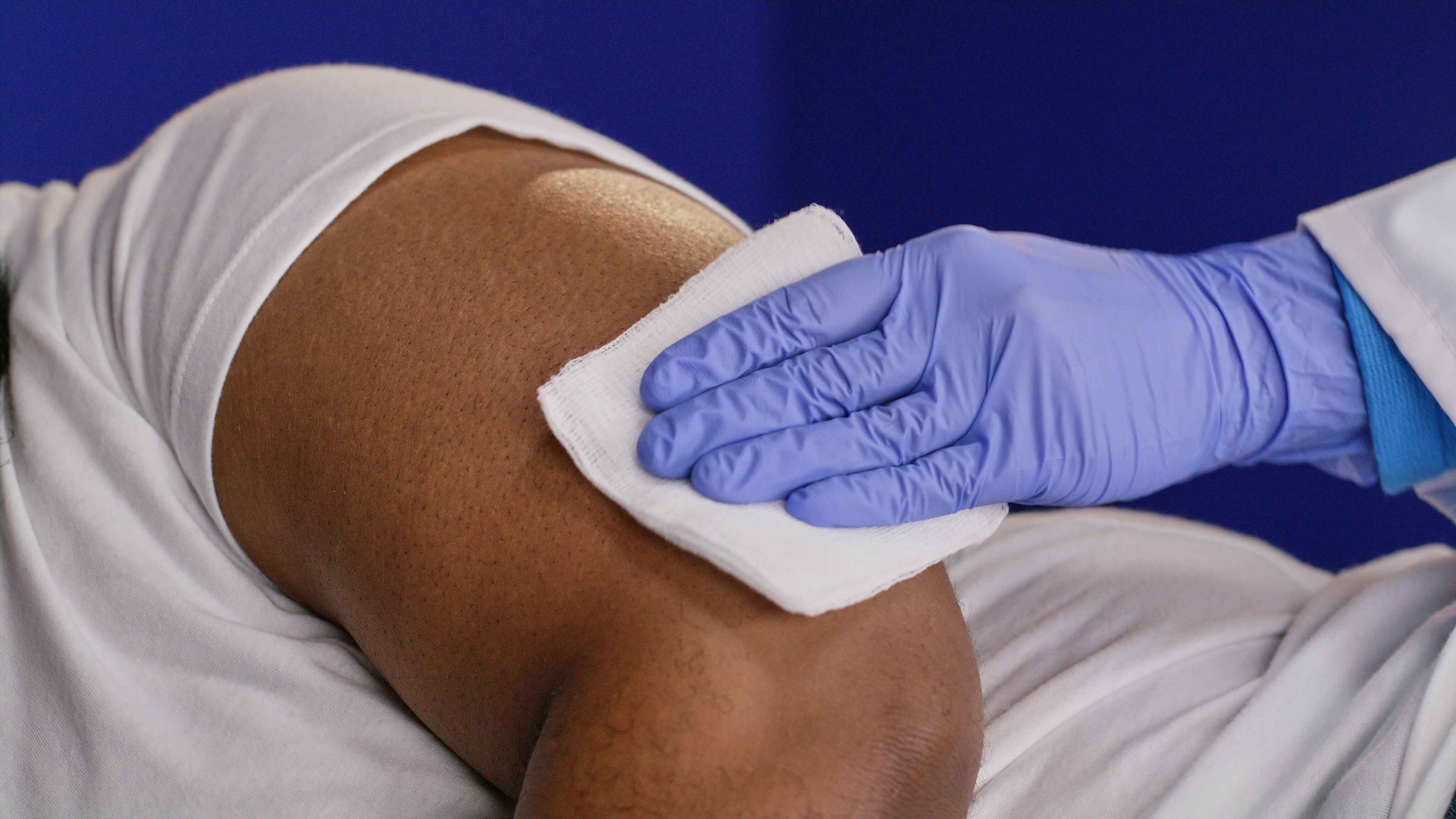
The provider sterilizes and numbs the area
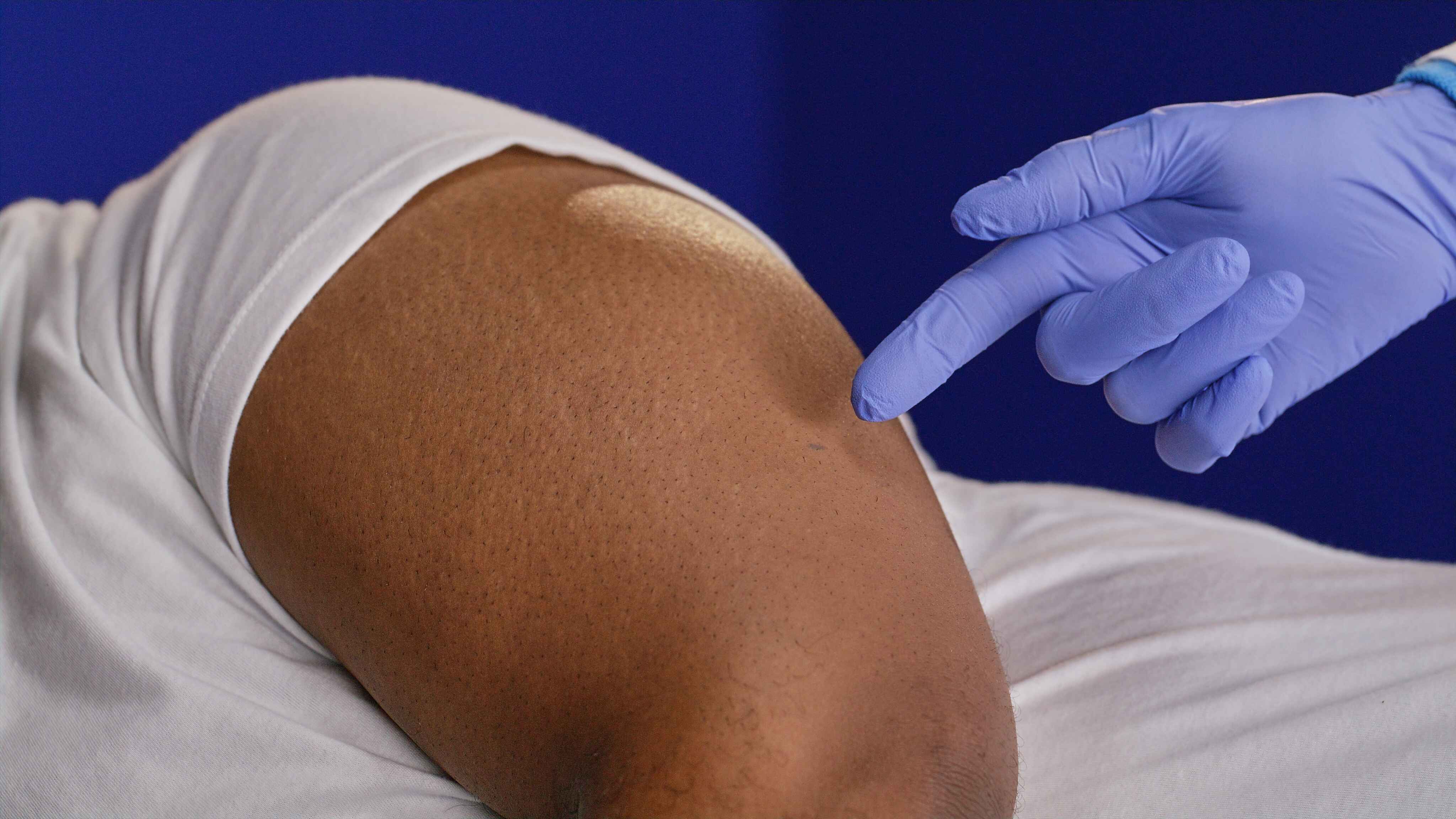
A tiny incision is made, just big enough to accommodate the sensor’s width
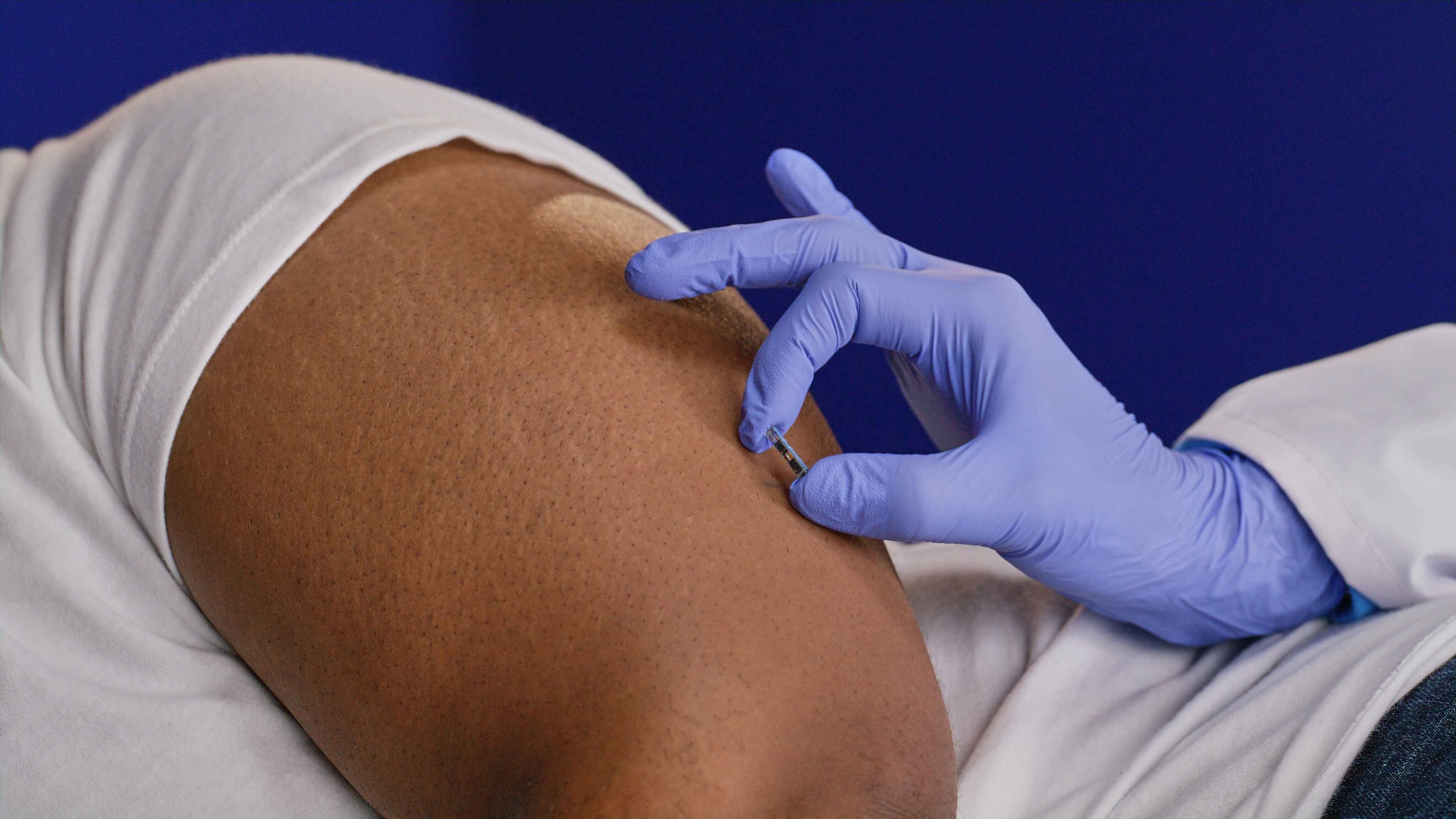
The provider makes a small pocket under the skin and inserts the sensor
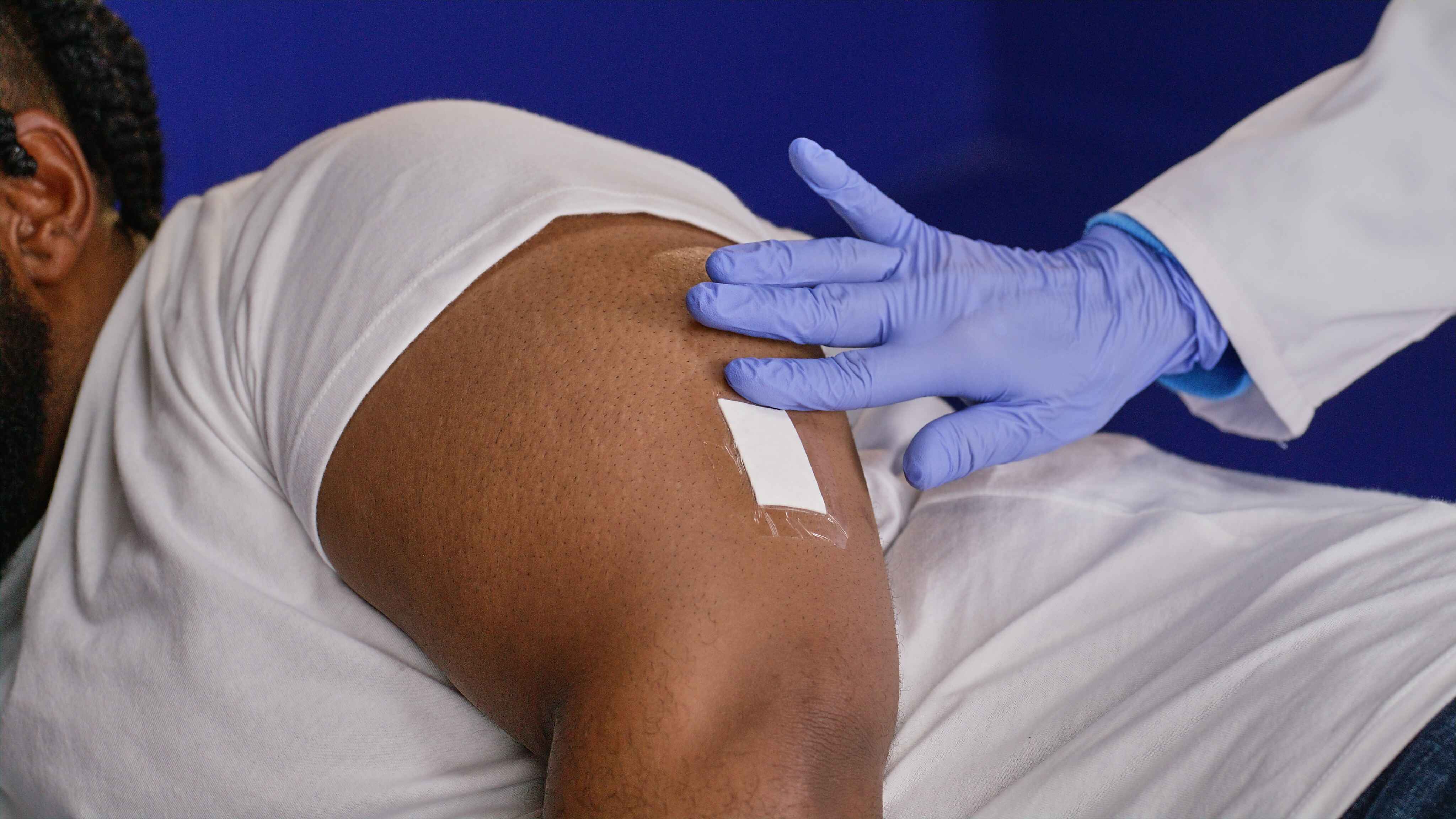
A few adhesives and a bandage are applied
After the sensor placement
The sensor, transmitter and Eversense mobile app get connected. You’ll have a 24-hour warmup, and then you can monitor your glucose safely and reliably for a YEAR.
After one year of monitoring glucose without the frustrations of a traditional CGM, you’ll return to the Eversense Inserter so they can take the old sensor out and put a new one in your other arm, same as before.
Ready to take the first step? Get pre-qualified.


For further discussion with your doctor
Your endocrinologist might not know about Eversense 365. We put everything they might like to know on one page.
Want to see if you
qualify?
Take our quick and easy quiz to see if Eversense 365 could work for you.
1. Senseonics. (2024) Eversense 365 Continuous Glucose Monitoring System User Guide. LBL-7702-01-001.
2. Bailey, T. S. et al. (2025). Evaluation of accuracy and safety of the 365-day implantable Eversense continuous glucose monitoring system: The ENHANCE study. Diabetes Technology & Therapeutics, 27(5), 407–411. https://doi.org/10.1089/dia.2024.0592
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. Ascensia, the Ascensia Diabetes Care logo are trademarks and/or registered trademarks of Ascensia Diabetes Care Holdings AG. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
PP-SENS-365-GBL-0187